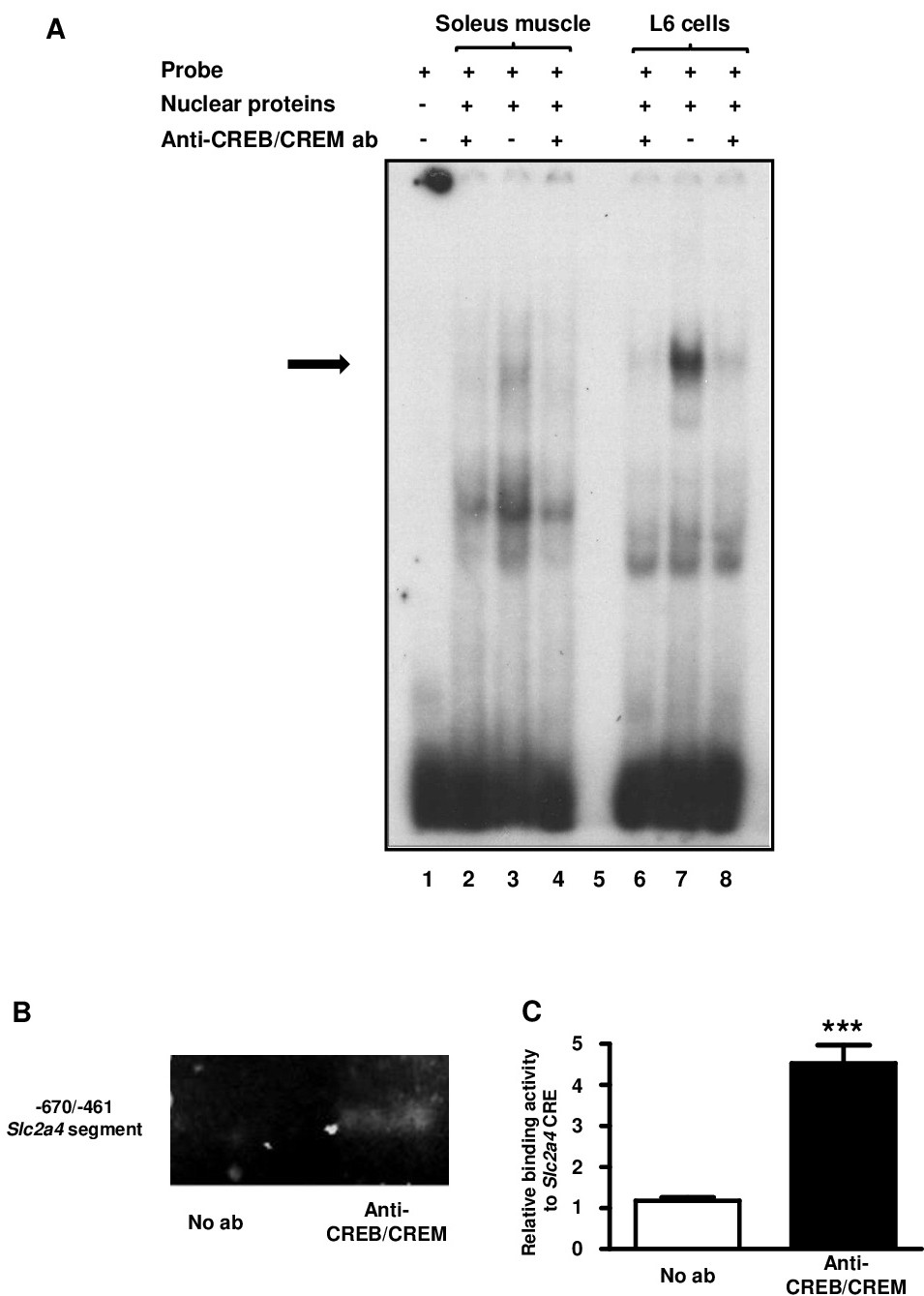
Fig. 4. Skeletal muscle CREB/CREM nuclear proteins bind selectively in the Slc2a4 CRE in vitro and in vivo. In vitro binding activity (A) of nuclear proteins from rat soleus and L6 muscle cells was analyzed by electrophoretic mobility shift assay, using the wild type CRE binding site of the rat Slc2a4 promoter as probe, and competitions were performed by adding 10Ág of anti-CREB/CREM antibody (CREB, cAMP responsive element binding protein; CREM, CRE modulator protein). In vivo CREB/CREM binding activity (B and C) into the CRE binding site of the rat Slc2a4 promoter gene was analyzed by chromatin immunoprecipitation (ChIP) assay. DNA was extracted from soleus muscle, fragmented, immunoprecipitated with anti-CREB/CREM antibody or not (No ab), and the -670/-461 sequence of Slc2a4 gene was amplified by qPCR. To confirm the efficiency of immunoprecipitation, the PCR products were electrophoresed on ethidium bromide-agarose gel (B) or quantified in the qPCR (C). A and B, representative images; C, mean ▒ SEM of samples from 3 animals, treated or not with antibody. ***P<0.001, unpaired two-tailed Student`s t-test.